Sodium Sulfate Anhydrous
Sodium Sulfate – Na2SO4
CAS# 7757-82-6
MW 142.02
ACS Grade
Additional information
Brand
Caisson Labs
Product Storage Conditions
15 to 30°C
Product Shipping Conditions
Ambient
Product Attributes
| Weight | N/A |
|---|---|
| Dimensions | N/A |
| Format | |
| Size |
SODIUM SULFATE
A commercially important inorganic salt and a raw material present in both hydrous and anhydrous forms. Sodium sulfate (Na2SO4) is widely used in paper, glass and textile industries [3].
INTRODUCTION
- Sodium sulfate (Na2SO4) was discovered by John Glauber in the 17th century that was named Sal mirabilis or Glauber’s salt after the name of a scientist [2].
- Sodium sulfate (Na2SO4) is equally recognized under the names of various labels in the market like Disodium sulfate, Sodium sulfate (anhydrous), Sodium sulfate dried [JAN], Natriumsulfat (German), Sodium tallow alcohol sulfate, Glauber’s salt, Sal mirabilis (decahydrate), salt cake, and Mirabilite [6].
- Sodium sulfate (Na2SO4) is available in the market in poly bottles of 500 grams and 1 kilogram. Bulk quantities like 2.5 kilograms, 5 kilograms, 12 kilograms, and 50 kilograms are obtainable in poly drums [7].
STRUCTURE
- The molecular structure of sodium sulfate (Na2SO4) is shown below [2].
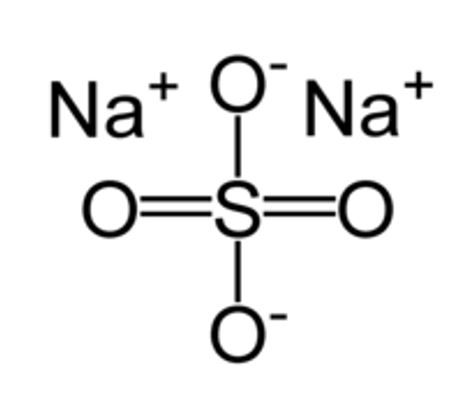 Figure 1: Structure of sodium sulfate [2].
Figure 1: Structure of sodium sulfate [2].
PROCEDURE
There are two ways of obtaining sodium sulfate (Na2SO4) that are described below [1][2].
- Natural Production of sodium sulfate (Na2SO4).
- Synthetic Production of sodium sulfate (Na2SO4).
- Natural Production of sodium sulfate (Na2SO4):
- Sodium sulfate is additionally obtained from natural resources in various forms combined with other minerals.
- Mirabilite and thenardite are two forms of sodium sulfate (Na2SO4) that are of commercial importance.
- Mirabilite is found naturally in the lake water beds of southern Saskatchewan and it may occur as a constituent of surface salt. The United States of America produces about 350,000 tons annually.
- Some other forms of sodium sulfate are also obtained from sources like glauberite (calcium sulfate is combined with sodium sulfate in this form) and blödite (magnesium sulfate is present along with sodium sulfate).
- Synthetic Production of sodium sulfate (Na2SO4):
- There are many processes used commercially for the production of sodium sulfate (Na2SO4). These processes are described below.
- Hargreaves process:
- A process of manufacturing sodium sulfate (Na2SO4) by passing a mixture of sulfur dioxide and air through aqueous brine (sodium chloride) in a countercurrent manner. This process is also followed for the commercial production of hydrochloric acid [4].
- In this process, the main constituents of the production of sodium sulfate are sulfur dioxide and an aqueous solution of sodium chloride (brine). Air is also needed in this process. During the production of Hydrochloric acid, sodium sulfate is also produced.
Chemical Reaction:The chemical equation of reaction that occurs during Hargreaves process is given below.
Sodium chloride + Sulfur dioxide + Air + water ➔ Hydrochloric acid + Sodium sulfate4NaCl + 2SO2 + O2 + 2H2O ➔ 4HCl + 2Na2SO4
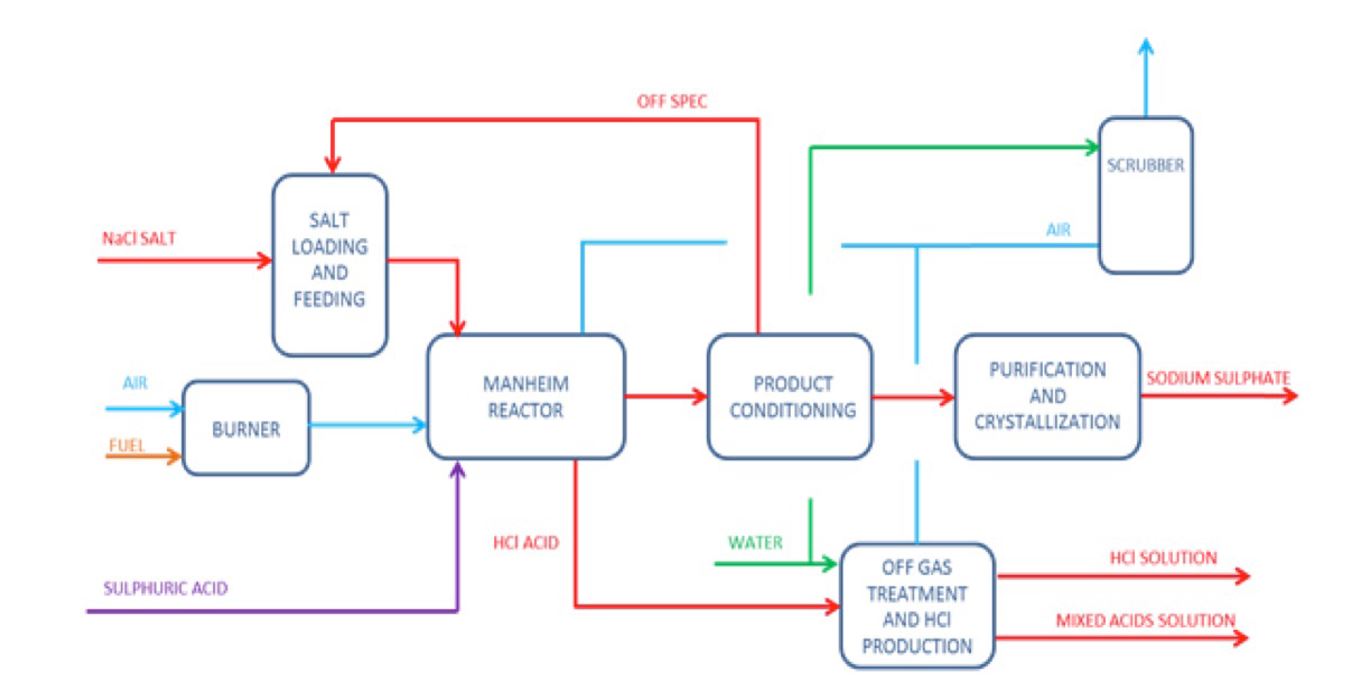
- Mannheim process:
- A process of manufacturing sodium sulfate (Na2SO4) by passing a mixture of sulfuric acid from sodium chloride solution (brine) that additionally results in the production of hydrochloric acid [5].
Chemical Reaction:
The chemical reaction that occurs during the synthesis of sodium sulfate (Na2SO4) in Mannheim’s process is given below.Sodium chloride + sulfuric acid ➔ hydrochloric acid + sodium sulfate
2NaCl + H2SO4 ➔ 2HCl + Na2SO4
- A process of manufacturing sodium sulfate (Na2SO4) by passing a mixture of sulfuric acid from sodium chloride solution (brine) that additionally results in the production of hydrochloric acid [5].
- The flow chart of Mannheim’s process is shown below [5].
Figure 2: Flow chart of Mannheim’s Process [5].
SPECIFICATIONS
The specifications of sodium sulfate (Na2SO4) are described below [4][7][8].
| PROPERTIES | Sodium Sulfate |
|---|---|
| State | Solid |
| Appearance | In form of crystals or powder |
| Color | White or Colorless |
| Nature | Hygroscopic |
| Odor | Odorless |
| Density |
|
| Molecular formula | Na2SO4 |
| Molecular weight | 142.04 grams per mole |
| Solubility |
|
| Solubility in water | 4.76 g/100 ml at zero degrees Celsius |
| Boiling point | 1429 degrees Celsius (anhydrous) |
| Melting point | 884 degrees Celsius (anhydrous) |
| Formal charge | 0 |
| Viscosity | 2.48 |
| On decomposition | Produce toxic fumes |
| Entropy | 149.6 J/mole. K |
| Storage temperature | Room temperature is suitable |
| CAS Number | 7757-82-6 |
| InChI | 1S/2Na.H2O4S/c;;1-5(2,3)4/h;;(H2,1,2,3,4)/q2*+1;/p-2 |
| InChI key | PMZURENOXWZQFD-UHFFFAOYSA-L |
| SMILES | [Na+].[Na+].[O-]S([O-])(=O)=O |
APPLICATIONS
The commercially and industrially important applications of sodium sulfate (Na2SO4) are described below [9] [10].
- Dry Organic Liquids:Sodium sulfate (Na2SO4) is used to dry out organic liquids.
- Washing Industry:
Sodium sulfate (Na2SO4) is an important component of the washing industry. It is used in the manufacturing of detergents and soaps. - Removal of Air Bubbles
It is additionally used in the glass industries from removing air bubbles from the glass. - Pharmaceutical Industry:
Sodium sulfate (Na2SO4) is also known as laxative Glauber’s salt. It is used as a laxative for cleaning the bowel from the colon. - Food Industry:
Sodium sulfate (Na2SO4) is used in the production of starch and also acts as an additive in the food for cattle. - Textile Industry:
It is used in the textile industries for leveling fibers.
SAFETY AND HAZARDS
Sodium sulfate is considered non-toxic chemical. The safety and hazards are described below [11] [12].
- Skin Contact: It can cause skin irritation, redness, itching, and allergic reactions on exposure.
First Aid Measure: Instantly remove the contaminated clothes. Wash the face and skin continuously for 15 to 20 minutes or until the irritation ends. Consult medical advice on the severity of symptoms. - Eyes Contact: It can cause irritation, redness, temporary and permanent damage to the eyes because of long-term exposure to powder or dust form.
First Aid Measure: Wash the eyes immediately with plenty of water for 10 minutes continuously. Seek medical advice. - Ingestion: In case of ingesting sodium sulfate, it can cause nausea, GIT infection, vomiting, and headache.
First Aid Measure: Do not induce vomiting. Do not give anything to drink or eat to the victim. Immediately move the person to fresh air. Seek medical advice. - Inhalation: In case of inhaling the mist, vapors, or powder of sodium sulfate, irritation of mucus membranes may occur. It can also lead to respiratory tract infections.
First Aid Measure: Immediately move the person to an uncontaminated place where he can breathe easily. Seek medical advice.
Safety Measures: Dispose of the remaining chemical carefully. Wear proper chemical-resistant clothes or lab coats to minimize contact with skin while dealing with sodium sulfate. Use protective goggles and gloves for preventing any kind of eye contact with the chemical. Use a face shield for preventing the splashes of sodium sulfate solution.
REFERENCES
- One Mine – Sodium Sulfate From Natural Sources
- McGill – Sodium Sulfate
- PubChem NCBI NIH – Sodium Sulfate
- Chem Europe – Sodium Sulfate
- Desmet Ballestra – Sodium Sulphate
- PubChem NCBI NIH – Sodium Sulfate 2.4.1 MeSH Entry Terms
- Sigma Aldrich – Sodium Sulfate
- PubChem NCBI NIH – Sodium Sulfate 3.2.3 Odor
- BYJU – Sodium Sulfate – Na2SO4
- Mayo Clinic – Sodium Sulfate, Potassium Sulfate, And Magnesium Sulfate (Oral Route)
- Fisher Scientific – Sodium Sulfate
- Lewis University – Sodium Sulfate Safety Data Sheet
| Product Lot Number: |
*All products for Laboratory use only