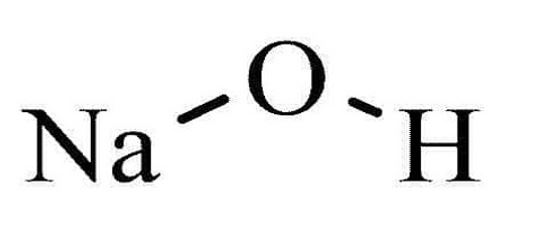Sodium Hydroxide – Pellets
CAS# 1310-73-2; MW 40
Additional information
Brand
Caisson Labs
Product Storage Conditions
15 to 30°C
Product Shipping Conditions
Ambient - Dangerous Good
Product Attributes
| Format | |
|---|---|
| Size |
SODIUM HYDROXIDE
A synthetically manufactured inorganic colorless liquid is also known as caustic soda. Sodium hydroxide efficiently absorbs adequate moisture from the surrounding atmosphere [5].
INTRODUCTION
- Sodium hydroxide formula is the most common and strong base used in the industry.
- It is obtainable in the market in the packaging of 25g, 100g, 250g, and 1kg packed in plastic bottles and containers. It is equally achievable in bulk quantity like 50kg in HDPE Drum [7].
- Sodium hydroxide formula is also obtainable under the names of various labels in the market like Sodium hydrate, Caustic soda, White caustic, Natrium hydroxyde, Liquid-plumr, Sodium hydroxide formula, Fuers Rohr, and Rohrreiniger Rofix [6].
STRUCTURE
- The structure of most common base (sodium hydroxide formula) is shown below.
Figure 1: Structure of NaOH [4].
PROCEDURE
Different methods are used for the production of sodium hydroxide that are discussed below [1][2].
- Castner – Kellener Process
- Nelson’s Method
- Loewig’s Process
- Gibb’s Method
- Nelson Diaphragm Cell
- Lesueur’s process
A) Castner – Kellener Process:
Here Castner Kellener Process is discussed in detail which results in the production of sodium hydroxide at industrial level.
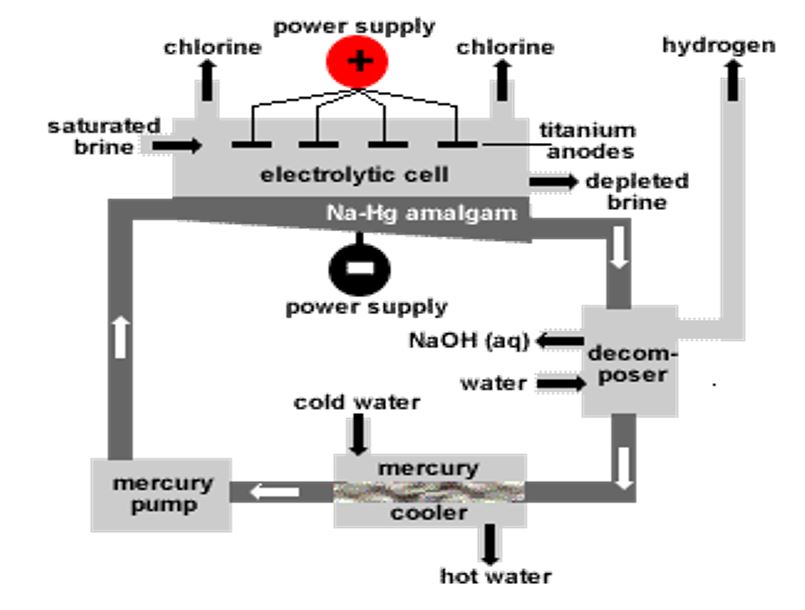
Castner Kellener Cell:
- In this process, a rectangular steel tank is used which is known as Castner Kellener cell. This cell is lined by ebonite (a type of rubber). This cell has a cathode made up of liquid mercury at the bottom of the tank and an anode made up of titanium [1].
- In this industrial process, sodium hydroxide formula (NaOH) is produced by the splitting of aqueous sodium chloride named as brine, by using electricity.
- The concentration of brine used in this process is made up by 25 grams of sodium chloride dissolved in 75 grams of water [2].
Figure 2: Castner Kellener Cell [1].
Production Process:
- The effective process in Castner Kellener cell is initiated when an electric current passed through the aqueous solution of sodium chloride (NaCl). Ionization occurs that results in the production of positive and negative ions in the aqueous solution. These ions moved toward their respective electrodes (cathode and anode).
- The sodium ions (Na+) moved towards the cathode (made up of mercury). At the cathode, sodium reacts with mercury and results in the production of sodium amalgam.
Reactions at cathode:2Na+ +2 e– ➔ 2Na
Na + Hg ➔ Na/Hg - The chlorine ions (Cl–) moves toward the anode. Following reaction occurs at the anode.
Reactions at anode:2Cl– ➔ Cl2 + 2e–
- Amalgam moves to denuder (another chamber). It is treated with water and results in the production of sodium hydroxide.
2Na/Hg + 2H2O ➔ 2NaOH + H2 + 2Hg
- Liquid sodium hydroxide is produced in this process. Solid sodium hydroxide can be obtained by the evaporation of this liquid. A highly pure product is formed at the end [1].
Specifications:
The specifications of sodium hydroxide are described below [4][9].
| PROPERTIES | Sodium Hydroxide Formula |
|---|---|
| Appearance | Available in Powder, Crystals or in the form of granules |
| State | Solid; Liquid |
| Color | White or Opaque Crystals; Colorless Liquid |
| Odor | Odorless |
| Nature | Hygroscopic |
| Molecular formula | NaOH |
| Exact Mass | 39.992508 |
| Molar mass | 39.9 grams per mole |
| Viscosity | 4.0 cP at 350 °C |
| Density | 2.13 grams per cm3 |
| Solubility | Soluble in water, ethanol, glycerol and methanol |
| Melting point | 323 °C |
| Boiling point | 1,388 °C |
| Solubility in water | 418 grams per Liter |
| Heat of vaporization | 175 kJ/mole |
| CAS Number | 1310-73-2 |
| CAS Number | 133-32-4 |
| InChl | 1S/Na.H2O/h;1H2/q+1;/p-1 |
| InChl key | HEMHJVSKTPXQMS-UHFFFAOYSA-M |
| SMILES | [OH-].[Na+] |
APPLICATIONS
Following are some applications of sodium hydroxide formula that are discussed below [3][8].
- Refining of oil: Sodium hydroxide is consumed in the refining of oil and petroleum.
- Paper industry: it is additionally used in the paper industry for the production and recycling of paper.
- Purification of Bauxite: Sodium hydroxide formula is additionally used in the purification procedure of Bauxite.
- Drain cleaner: Sodium hydroxide is used to unclog the pipelines, and that’s why it acts as a drain cleaner.
- Cleansing Agent: It also acts as a cleansing agent and is used in the washing procedures of metallic sheets. It is used in soaps, detergents, and oven cleaners.
- Pharmaceutical industry: Sodium hydroxide formula is used as a blood coagulant (for prevention of blood flow), in cholesterol-reducing medicines, and pain reliever.
- Canning processes: Sodium hydroxide formula is used in the canning of food and is additionally used to peel off the skin of vegetables.
SAFETY AND HAZARDS
The safety and hazards are discussed below [10] [11].
- Corrosive in nature: Sodium hydroxide formula is potentially corrosive in nature. The effect of caustic acid depends on the time of exposure, and concentration of NaOH. It is corrosive for metals.
- Contact with eyes: sodium hydroxide formula is dangerous for the eyes. Long-term exposure of powder to the eyes can cause serious damage. Try to protect unexposed eyes. It can cause irritation and severe infection.
First Aid Measure:
If eyes get in contact with the liquid or powder form, wash with plenty of water for 15 to 20 minutes. Open upper and lower eyelids and wash them gently with splashes of water. In case of severe irritation, consult medical advice immediately.
- Contact with skin: sodium hydroxide is corrosive for the skin. If it gets in contact with the skin, it can cause discomfort, redness, irritation, or even burn the skin from the place of contact.
First Aid Measure:
Immediately remove the contaminated clothes of the victim. Wash the affected area thoroughly from plenty of water. Use neutral soaps or liquid soaps for washing the body parts. Wash the affected areas for 15 to 10 minutes.
- Inhalation: Sodium hydroxide formula can cause irritation and burning sensation in the membranes of the respiratory system on inhalation of powder, vapors, or dust of sodium hydroxide.
First Aid Measure:
Immediately move the person to fresh air. Lose his clothes so that he can breathe easily. Consult medical advice.
- Ingestion: If it is ingested mistakenly, it can cause nausea, irritation, and pain in the abdomen.
First Aid Measure:
Do not induce vomiting. Immediately wash the mouth of the victim with excess water. Consult medical advice.
- Safety measures: Wear safety goggles, gloves, and protective chemical resistant clothes. Use well-ventilated areas for experimentation and research purposes.
REFERENCES
- World of Chemicals – Methods of preparation of caustic soda
- City Collegiate – CHEMISTRY OF SODIUM HYDROXIDE
- TOPPR – Sodium Hydroxide – Preparation, Properties and Uses
- Assignment Point – Sodium Hydroxide – a Chemical Compoun
- PubChem NCBI NIH – Sodium hydroxide
- PubChem NCBI NIH – Sodium hydroxide 2.4.2 Depositor-Supplied Synonyms
- India Mart – Sodium Hydroxide Pellets
- Department of Health – Sodium Hydroxide (NaOH) – What are some uses of sodium hydroxide?
- PubChem NCBI NIH – Sodium hydroxide 3. Chemical and Physical Properties
- Department of Health – Sodium Hydroxide (NaOH)
- Fisher Scientific – Sodium Hydroxide Safety Data Sheet
| Product Lot Number: |
There’s more in our box!
*for Laboratory use only