SODIUM PHOSPHATE DIBASIC ANHYDROUS
A hygroscopic white powdery sodium salt of phosphoric acid that is used as a laboratory chemical in manufacturing of the number of products [1].
1. Introduction:
- Sodium phosphate dibasic anhydrous is a powdery or crystalline salt that can absorb water from the air. That’s why it is also known as hygroscopic salt.
- It is also stocked under the names of different labels in the market like Disodium hydrogen phosphate, Phosphate of soda, Disodium hydrogen orthophosphate, Disodium acid orthophosphate, Caswell No. 778, Buffer Salt, pH 6.87, Sodium phosphate dibasic, Trace metals grade, Sodium mono hydrogen phosphate (2:1:1), and, Sodium phosphate, dibasic, 99+%, ACS reagent, suitable for buffer solutions [5].
- It is obtainable in the market in various sizes like in the packaging of 100 grams, 250 grams, 500 grams, 1 kilogram, and 2.5 kilograms [6].
- It is typically packed in poly bottles tightly sealed. But if its quantity is 2.5 kg, it is packaged in poly drums [6].
2. Structure:
The molecular structure of sodium phosphate dibasic anhydrous is shown below [2].
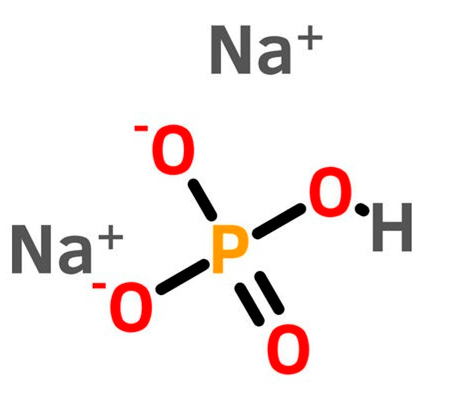
Figure 1: Structure of sodium phosphate dibasic anhydrous [2].
3. Procedure:
There are many methods that are used for the production of sodium phosphate dibasic anhydrous. Some of them are described below [4].
- Phosphoric Acid with Sodium Carbonate: Sodium phosphate dibasic anhydrous can be prepared by the following method.
The solution of phosphoric acid is treated with soda ash (sodium bicarbonate). First of all, the mixture of phosphoric acid and soda ash is heated until it boils and starts expelling carbon dioxide. After the release of carbon dioxide, the solution is cooled down and dodecahydrate crystal formation occurs. Now again by heating dodecahydrate at 100 degrees Celsius, anhydrous salt is formed.
Chemical equation:
H3PO4 + Na2CO3 à Na2HPO4 + CO2 + H2O - Dibasic Calcium Phosphate with Sodium Carbonate: Sodium phosphate dibasic anhydrous is prepared by the reaction between dibasic calcium phosphate and sodium carbonate. While treating them together, calcium carbonate precipitates in the bottom of the container leaving dibasic sodium salt behind. On cooling, the solution produces anhydrous dibasic sodium phosphate.
Chemical equation:
CaHPO4 + Na2CO3 à CaCO3 + Na2HPO4
4. Specifications:
The specifications of sodium phosphate dibasic anhydrous are described below [3] [4] [6].
| Properties | Sodium Phosphate Dibasic Anhydrous |
| Physical State | Solid |
| Appearance | Powdery or crystalline |
| Color | White or colorless |
| Odor | Odorless |
| Taste | Saline |
| Molecular Weight | 141.96 |
| Grade | Anhydrous |
| Density | 1.064 g/mL |
| Nature | Hygroscopic |
| Stability | Chemically stable |
| pH | 8.9 – 9.2 |
| Log P | 5.8 |
| Formal charge | 0 |
| Vapor density | 4.9 |
| Solubility | Soluble in water Insoluble in alcohol |
| Solubility in water | Almost equals to 10 g/100 mL at 20 degrees Celsius |
| Decomposition | Decompose at ~250 °C |
| Specific gravity | 2.066 |
| Storage temperature | 5°C – 30°C |
| InChI | 1S/2Na.H3O4P/c;;1-5(2,3)4/h;;(H3,1,2,3,4)/q2*+1;/p-2 |
| InChI Key | BNIILDVGGAEEIG-UHFFFAOYSA-L |
| SMILES | OP(=O)([O-])[O-].[Na+].[Na+] |
5. Applications:
The applications of sodium phosphate dibasic anhydrous are described below in detail [9] [10].
- Adjust pH:
Sodium phosphate dibasic anhydrous is used in adjusting or maintaining the pH of the solution. It acts as a buffer. - Food Products:
Phosphate of soda is used as an emulsifier in food products. It is used in the thickening of food constituents. - Starch Production:
Sodium phosphate dibasic anhydrous is additionally used in the production of starch. - Fragrance ingredient:
It is also used as an essential ingredient in some fragrances. - Household Products and Cleaning Agents:
Sodium phosphate dibasic anhydrous is used in many household products like Body cleaners, hand washes, shower gels, hand and body moisturizers. It is also used as a cleaning agent as a constituent of detergents and soaps. - Personal care:
It is also used in many personal care products like shampoos and conditioners. - Medical Industry:
It is used widely in medical and dental industries. It is used as a laxative (a medicine to treat the constipation and to clean bowel from the colon). - Production of ceramics and enamel:
It is used in the production of ceramics and enamel.
6. Hazards and Safety Measures:
Sodium phosphate dibasic anhydrous is not considered hazardous but it can cause many health-related issues on long-term exposure. The hazards and safety measures are of sodium phosphate dibasic anhydrous are described below [7] [8].
Health Effects: Following are the health effects that are caused by sodium phosphate dibasic anhydrous that are described below.
- Eyes Exposure to sodium phosphate dibasic anhydrous:
If eyes are exposed inadvertently to the powdered form of sodium phosphate dibasic anhydrous, it can cause itching, irritation, and redness.
First Aid Measure:
Carefully wash the eyes with plenty of water for 15 to 20 minutes. In case of pain and irritation, consult the doctor immediately. - Skin Exposure to Sodium phosphate dibasic anhydrous:
In case of skin exposure to the powdered form or solution, it can cause skin rash, itching, and irritation. Prolonged exposure can cause severe dermatitis on the skin.
First Aid Measure:
Promptly remove the clothes that get contaminated by sodium phosphate anhydrous. Wash the body parts and clothes with plenty of water. Use neutral soap for washing purposes. The washed clothes can be re-used. - Accidentally Ingested:
Sodium phosphate dibasic anhydrous on ingestion can cause nausea, a headache, and disturbance in the digestive system.
First Aid Measure:
Don’t offer anything to consume or drink to the victim. Instantly assist the victim to the hospital so that he can be handled accordingly. - Inhalation:
On inhalation, it can cause respiratory tract infections which can lead to severe health problems on long-term exposure like coughing, irritation in the nose, and wheezing.
First Aid Measure:
Carefully influence the victim to a place where fresh air is available. Carry out artificial breathing, if the victim is not breathing. - Personal Precautions:
Avoid dust, vapor, or mist inhalation of sodium phosphate dibasic anhydrous. Prevent any type of bodily contact with the powder or liquid form. Use proper protective equipment (gloves, lab coat, and goggles). - Recommendations:
- UseSodium phosphate dibasic anhydrous, after reading the expiry date on the sealed container.
- Don’t usethe product (in powder or liquid form) if it is expired.
- Take care of the storage conditions of the solution/powder. Use the product in aseptic conditions to limit possible contaminations.
7. References:
- https://www.mpbio.com/sodium-phosphate-dibasic-anhydrous-usp
- https://www.spectrumchemical.com/sodium-phosphate-dibasic-anhydrous-fcc-s1402#shipping.storage
- https://pubchem.ncbi.nlm.nih.gov/compound/Disodium-hydrogen-phosphate#section=Chemical-and-Physical-Properties
- https://www.chemicalbook.com/ChemicalProductProperty_EN_CB1242667.htm
- https://pubchem.ncbi.nlm.nih.gov/compound/Disodium-hydrogen-phosphate#section=Depositor-Supplied-Synonyms
- https://www.sigmaaldrich.com/PK/en/product/sigma/rdd022
- https://www.fishersci.com/store/msds?partNumber=BP3321&productDescription=SOD+PHOSPHATE+DIBAS+ANHY+1KG&vendorId=VN00033897&countryCode=US&language=en
- https://www.sigmaaldrich.com/PK/en/sds/sigma/rdd022
- https://nj.gov/health/eoh/rtkweb/documents/fs/1723.pdf
- https://pubchem.ncbi.nlm.nih.gov/compound/Disodium-hydrogen-phosphate#section=Uses









