Description
TRIS HCl – C4H12CINO3 Tris Buffer solution used extensively in the field of Molecular Biology.

Molecular Formula:C4H12CINO3 CAS# 1185-53-1; MW 157.6
TRIS HCl – C4H12CINO3 Tris Buffer solution used extensively in the field of Molecular Biology.
Caisson Labs
15 to 30°C
Ambient
| Format | |
|---|---|
| Size |
A Tris Buffer solution has two formulations a Tris Base formulation and a Tris HCl formulation. The only difference between the two is hydrochloric acid added to Tris Base to create Tris HCl. Tris base (C4H11NO3) is an effective buffer for biological processes, which is why it is also known as a Tris Buffer. Tris HCl (C4H12ClNO3) is almost equally an important component and used extensively for molecular biology. Tris HCl is normally more expensive than Tris base but they are both known as Tris Buffer solutions.
INTRODUCTION
STRUCTURE
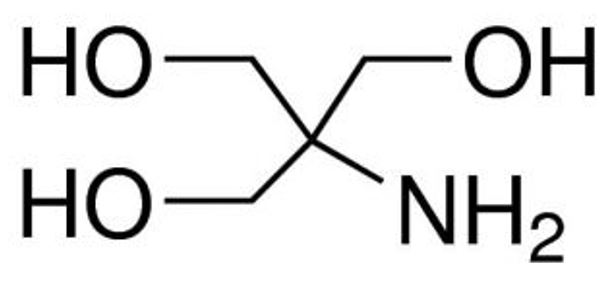 Figure 1: Structure of Tris-base [1]
Figure 1: Structure of Tris-base [1]
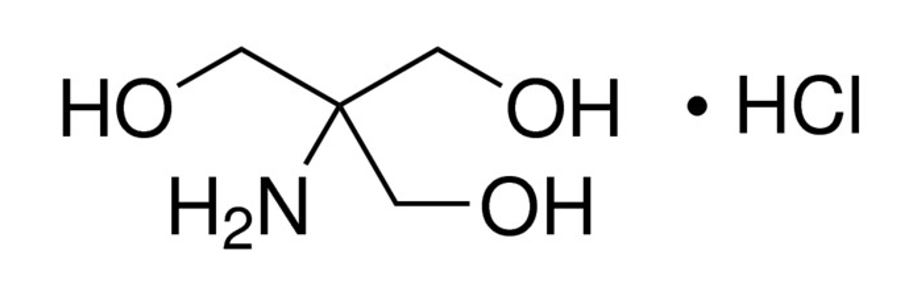 Figure 2: Structure of Tris HCl [2]
Figure 2: Structure of Tris HCl [2]
PROCEDURE
The procedures for the preparation of tris buffer, both tris base and tris HCl are described below [9] [10].
Preparation method of Tris Buffer:
To prepare tris buffer in laboratory, following material and chemicals are needed.
Required Chemical:
The chemicals required for the preparation are:
Required Material:
The laboratory material needed for the preparation are:
Preparation Steps:
SPECIFICATIONS
The specifications of tris buffer, both tris base and tris HCl are described below in the table [4] [5] [6] [8].
| PROPERTIES | Tris Base | Tris HCl |
|---|---|---|
| Physical state | Solid | Solid |
| Appearance | Crystalline powder | Crystals or Powder form |
| Color | White | Colorless |
| Molecular weight | 163.21 grams per mole | 157.59 grams per mole |
| Nature | Hygroscopic | Hygroscopic in nature |
| Density | 1,353 g/cm3 | 1.05 g/mL |
| Molecular formula | C7H17NO3 | C4H12ClNO3 |
| Solubility | Soluble in water | Soluble in water |
| Solubility in water | 550 g/L | 667 mg/ml |
| Stability | Stable compound | Stable compound |
| Boiling point | 219-220 °C | 357° C at 760 mmHg |
| Melting point | 167-172 °C | 150-152 °C |
| Flash point | 219-220 °C | N/A |
| pH | 10.5-12.0 Range: 7 – 9 | 2.5-4.0 Range: 7 – 9 |
| Storage temperature | 20-25 °C | Stored at room temperature |
| InChl | 1S/C7H17NO3/c8-7(1-4-9,2-5-10)3-6-11/h9-11H,1-6,8H2 | 1S/C4H11NO3.ClH/c5-4(1-6,2-7)3-8;/h6-8H,1-3,5H2;1H |
| InChl Key | GKODZWOPPOTFGA-UHFFFAOYSA-N | QKNYBSVHEMOAJP-UHFFFAOYSA-N |
| SMILES | C(CO)C(CCO)(CCO)N | C(C(CO)(CO)N)O.Cl |
APPLICATIONS
The applications of tris base and tris HCl are described below [11] [12].
SAFETY AND HAZARD:
The hazards and safety measures of a tris buffer is explained below [13] [14].
Health Effects: Following are the health effects that are caused by tris bases and tris HCl that are described below.
A) Eyes Exposure to Tris base and Tris HCl:
If eyes are exposed inadvertently to the powdered form or to the splashes of stock solution, it can be harmful to the eyes and cause itching, irritation, and redness. It can also cause temporary damage to the eyes.
First Aid Measure:
Carefully wash the eyes with excess water for 10 to 15 minutes. In case of severe irritation and pain, consult medical advice immediately.
B) Skin Exposure:
In case of skin exposure, skin rash, itching and irritation may occur on the contaminated body part.
First Aid Measure:
Promptly remove the contaminated clothes. Wash the clothes properly so that no trace of chemicals is left on them. Wash the skin with plenty of water for 15 to 20 minutes. Use neutral soap for washing purposes.
C) Accidentally Ingested:
Tris bases and Tris HCl on ingestion can cause disturbance in the digestive system and may be harmful.
First Aid Measure:
Don’t offer anything to eat or drink to the unconscious victim. Instantly assist the victim to the hospital so that he can be handled accordingly.
D) Inhalation:
On inhalation, it can cause respiratory tract infections which can lead to severe irritation or itching problems in the upper respiratory tract.
First Aid Measure:
Carefully move the victim to a spacious place where fresh air is available. Give artificial breathing, if the victim is not breathing.
REFERENCES
| Product Lot Number: |