Sodium Pyruvate Solution
100 mM solution (11 mg/ml) in purified water. Sterile filtered.
**Out of Stock- lead time 6-8 weeks**
Additional information
Brand
Caisson Labs
Product Storage Conditions
2 to 8°C
Product Shipping Conditions
Refrigerated
Product Attributes
| Weight | N/A |
|---|---|
| Dimensions | N/A |
| Format | |
| Size | |
| Availability Date |
SODIUM PYRUVATE
Sodium Pyruvate acts as an endogenous supplement that provides an additional energy source for growing cells in the culture [3].
- Sodium pyruvate is substantially a salt formed from the conjugated anion of pyruvate which is also known as pyruvic acid. It plays an extremely significant role in many metabolic pathways and chemical reactions [6].
- Molecular formula of sodium pyruvate is CH3 It is monoclinic in nature [7].
- Sodium pyruvate is also marketed on the distinctive names of various registered labels. For Example, pyruvic acid sodium salt, Guna-flam, sodium 2-oxopropanoate, pyruvate sodium, POD38AIF, 2-Oxopropanoic acid sodium salts, and many other names are additionally used [5].
- Pyruvic acid sodium salt is an organic salt which contain sodium. In other words, we can say that a sodium is attached with pyruvate. It is composed of one sodium atom, three hydrogen and three oxygen atoms covalently bonded with three carbon atoms.
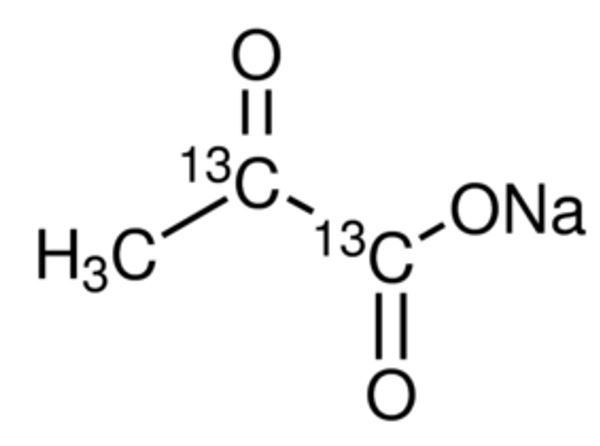
Figure 1: Structure of Sodium Pyruvate in powder form[2].
- Sodium pyruvate is used in cell culture media in addition to glucose because it is substantial in providing carbohydrate sources to developing cells under suitable conditions. It is added inside the growth media to enhance the survival rate of cells.
- It is not used in all cell culture mediums. Mostly the concentration of sodium pyruvate used in cell growth cultures is 1mM.
- It is also an intermediate in alcoholic fermentation and sugar metabolism where sodium pyruvate is converted into carbon dioxide and acetaldehyde by enzyme carboxylase.
- It is available in the packaging of 5g, 10g, 25g, 100g, 250g, and 500g [9].
PROCEDURE:
- Pyruvic acid holds a significant place in the chemical metabolisms of proteins and carbohydrates. It is consumed efficiently in numerous experimental processes related to physiology including studies on chemical reactions and metabolic pathways followed by rapidly growing tumor cells development.
- The most stable salt (sodium salt) bonded well with pyruvic acid and after bonding under suitable conditions it forms substantial and stable sodium pyruvate at room temperature.
- Following steps are carefully taken for the specific preparation of sodium pyruvate that is discussed below:
- Foremost, a Small quantity of pyruvic acid is prepared by mixing transparent crystalline tartaric acid and an inorganic compound named potassium hydrogen sulfate on a gentle heat. This mixing is typically done by the catalytic oxidation of a colorless liquid containing two alcohol groups in it, propylene glycol by a purplish-black salt potassium permanganate.
- This freshly prepared pyruvic acid is then neutralized with dilute alkali which is undoubtedly a time-consuming process and frequently produces contaminated condensed products.
- It is more adequate to accurately use alcoholic alkali for the neutralization of pyruvic acid because sodium pyruvate is insoluble in alcohol and can be removed from the reaction immediately after its formation.
- This method permits the hasty preparation of sodium pyruvate salt.
- In the laboratory, 10 ml of pyruvic acid is dissolved in 100 ml of alcohol. This selected sample stood for some weeks at normal room temperature, repeated distillations are not needed. After the required time, sodium pyruvate of good quality appeared prominently in the selected sample.
- Another method of preparing sodium pyruvate is by neutralizing pyruvic acid with alcoholic alkali which is produced by the dilutions of sodium hydroxide with alcohol. This organic reaction is also carried out at room temperature. After 24 hours, a white solid amorphous powder precipitates are typically formed in the chosen sample, which is sodium pyruvate [8].
APPLICATIONS:
- Sodium pyruvate produces protective effects against apoptosis (cell death) mediated by concentrated hydrogen peroxide.
- It is used in many experiments involving chemical reactions related to cell cultures to provide more energy to the specific growing cells [11].
- It is an antioxidant and free radical (reactive oxygen radical) scavenger. Sodium pyruvate scavenges oxygen radicals by oxidative phosphorylation and this reaction results in the production of carbon dioxide, acetate, and water. Due to this ability, sodium pyruvate suppresses the renal injury caused by hydrogen peroxide at the cellular level.
- Sodium pyruvate is additionally used as an additive in many food items.
- It is an effective anti-inflammatory agent. For Example, it inhibits the adverse effects of acute paw edema knowingly caused by carrageenan in rats [10].
SPECIFICATIONS:
Following are the specifications of sodium pyruvate that are given below [1][2][3].
| Properties | Sodium Pyruvate |
|---|---|
| Color | Ranges from colorless to faint yellow color |
| Appearance Form | Available in both liquid and dry powder form |
| Odor | Slightly vinegar like |
| Solubility | 100mg/mL |
| Molecular Weight (g/mol) | 110.04 |
| Delivery Condition | Delivered at room temperature |
| Storage temperature | Ranges between 2 to 8 Degrees Celsius |
| Solubility | Soluble in H2O |
| Loss on drying | Less than or equals to 0.5% |
| Melting point | <300 Degrees Celsius |
| pH | 6.5-7.5 |
| Shelf life | 12 months almost from date of production |
| Parent Compound | Pyruvic acid |
| CAS Number | 113-24-6 |
| EC Number | 204-024-4 |
| H-Bond donor count | 0 |
| H-Bond acceptor count | 3 |
| Isotope count | 0 |
| Heavy atom count | 7 |
| Canonical SMILES | CC(=O)C(=O)[O-].[Na+] |
| InChl Key | DAEPDZWVDSPTHF-UHFFFAOYSA-M |
| InChl | 1S/C3H4O3.Na/c1-2(4)3(5)6;/h1H3,(H,5,6);/q;+1/p-1 |
HAZARDS & SAFETY:
- Sodium pyruvate is justly considered a hazardous material because it typically acts as an irritant and undoubtedly affects the individual both by ingestion and inhalation.
- It can invariably cause evident irritation in the eyes, in the unusual cases of direct contact immediately wash your unaided eyes with sufficient water and cleanse them thoroughly.
- Sodium pyruvate can also contaminate the clothes and cause serious irritation on the intact skin, in such tragic cases shed the contaminated clothes immediately and wash the affected area of your body with an excess amount of water [4].
- If it is ingested mistakenly, immediately induce vomiting and wash the victim’s mouth with water.
- In case of inhalation, directly transfer the victim to fresh air as a first-aid measure.
- Room temperature is carefully maintained because in case of decomposition of sodium pyruvate on heating it emits fumes.
- Ensure proper ventilation in constricted and confined places or laboratories.
- It is necessary to use chemical resistant protective clothes, gloves and goggles.
- After handling sodium pyruvate must wash your hands and face with water. Also, properly clean your used clothes [4].
References:
- PubChem – National Library of Medicine – Sodium Pyruvate Compound 1.2 3D Conformer
- Sigma-Aldrich – Sodium Pyruvate
- Gibco – Sodium Pyruvate
- ScholAR Chemistry – Sodium Pyruvate Material
- PubChem – National Library of Medicine – Sodium Pyruvate Compound 2.4 Synonyms
- The Importance of Sodium Pyruvate in Assessing Damage Produced by Hydrogen Peroxide
- The Crystal Structure of Sodium Pyruvate
- THE PREPARATION OF SODIUM PYRUVATE
- Sodium Pyruvate CAS 113-24-6
- What is Sodium Pyruvate
- The Applications of Sodium Pyruvate
| Product Lot Number: |
*All products for Laboratory use only